ABOUT US
Wiltrom Co., Ltd. is established in December of 2009, and it is a spin-off of Industrial Technology Research Institute. Wiltrom is dedicated in the research and production of a wide range of various high-end, implantable, Class II and III medical devices. The development of products is strongly focused on innovation and to create a highly competitive private brand on the market internationally.
Our company not only has the top researchers, but also is provided with an excellent working environment for increasing efficiency and competitiveness. Our GMP plant can manufacture and supply the best and latest medical devices.
Our value: Creating a better life for humanity with innovation, trust, and health.
WILTROM CORE COMPETENCE
Pioneering Innovation
Wiltrom is a leading provider of minimum invasive spine surgery solutions, dedicating 30% of our team to R&D. We integrate material science, mechanical engineering, and cutting-edge manufacturing to develop breakthrough solutions.
- 50+ patents across four major product lines
- Rapid commercialization from concept to clinical application
Manufacturing Excellence
Wiltrom operates a fully integrated production system, ensuring both scalability and precision to meet market demands.
- Scalable Production: Rapid production capacity expansion to meet global demand.
- High-Precision Manufacturing: To ensure superior accuracy and consistency.
- Strict Quality Control: Multi-stage inspections and ISO 13485-certified processes guarantee safety, reliability, and compliance with global regulations.
Quality Assurance
Wiltrom meets international medical standards, ensuring reliability and safety.
- Quality systems: ISO 13485, QMS, BGMP
- Regulatory Approvals: FDA (USA), CE (Europe), NMPA (China), TFDA (Taiwan), INVIMA (Colombia)…and others to come
PRODUCT INTRODUCTION
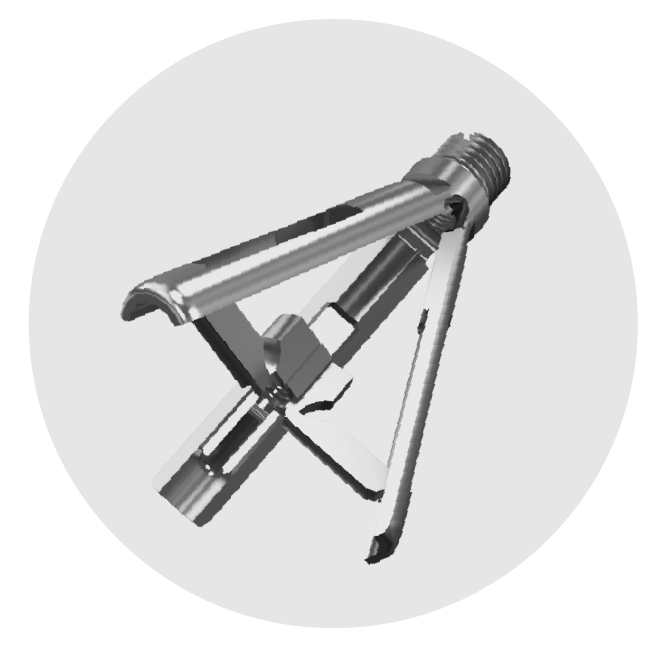
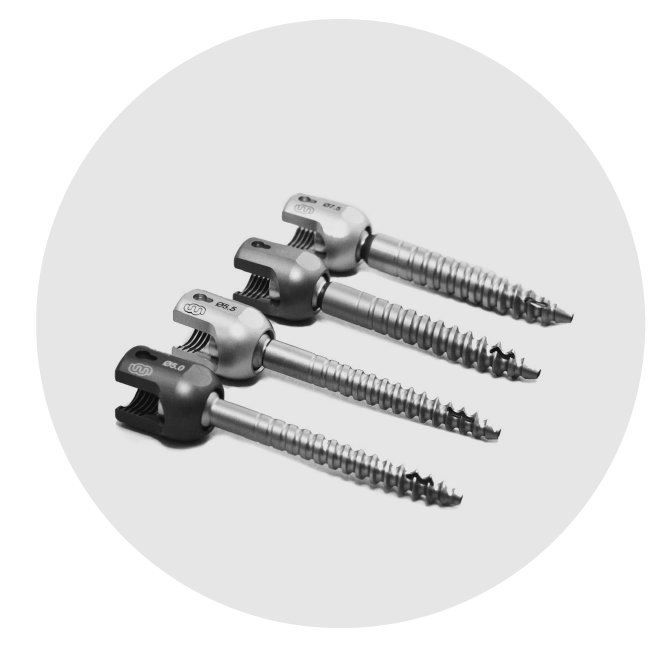
TRIPOD-FIX
TRIPOD FIX SALES OVERVIEW
Wiltrom is committed to developing innovative spinal surgical solutions, transforming concepts into clinical applications with high-quality medical devices. Wiltrom has specialized in advanced spinal surgical solutions, combining cutting-edge R&D, precision manufacturing, and world-class quality standards to improve patient outcomes globally.
Countries
Hospitals
Cases
Implants
Countries
Hospitals
Cases
Implants
NEWS
We would like to extend our sincere thanks to everyone who visited us during the 𝗘𝘂𝗿𝗼𝗦𝗽𝗶𝗻𝗲 𝟮𝟬𝟮𝟱 in Copenhagen, Denmark . It
We sincerely thank all healthcare professionals, partners, and industry colleagues who connected with us during this year’s exhibition in Bangkok. It was
TripodFix took the stage at the 𝗧é𝗰𝗻𝗶𝗰𝗮𝘀 𝗠𝗼𝗱𝗲𝗿𝗻𝗮𝘀 𝗲 𝗔𝘃𝗮𝗻ç𝗼𝘀 𝗱𝗮 𝗖𝗶𝗿𝘂𝗿𝗴𝗶𝗮 𝗱𝗮 𝗖𝗼𝗹𝘂𝗻𝗮 𝗩𝗲𝗿𝘁𝗲𝗯𝗿𝗮𝗹, held on August 29–30, 2025 in Ribeirão
On 𝗔𝘂𝗴𝘂𝘀𝘁 𝟵, 𝟮𝟬𝟮𝟱, Wiltrom, in partnership with 𝐇𝐨 𝐂𝐡𝐢 𝐌𝐢𝐧𝐡 𝐔𝐧𝐢𝐯𝐞𝐫𝐬𝐢𝐭𝐲 𝐨𝐟 𝐌𝐞𝐝𝐢𝐜𝐢𝐧𝐞 𝐚𝐧𝐝 𝐏𝐡𝐚𝐫𝐦𝐚𝐜𝐲 𝐇𝐨𝐬𝐩𝐢𝐭𝐚𝐥, successfully hosted the International Symposium
Wiltrom Co., Ltd. is conducting a prospective multi-center Post-Market Clinical Follow-Up (PMCF) study across Germany for its innovative vertebral body augmentation system,
We sincerely thank all the spine and neurosurgeons, educators, and participants who made the international cadaveric workshop at the CLEMI Foundation in
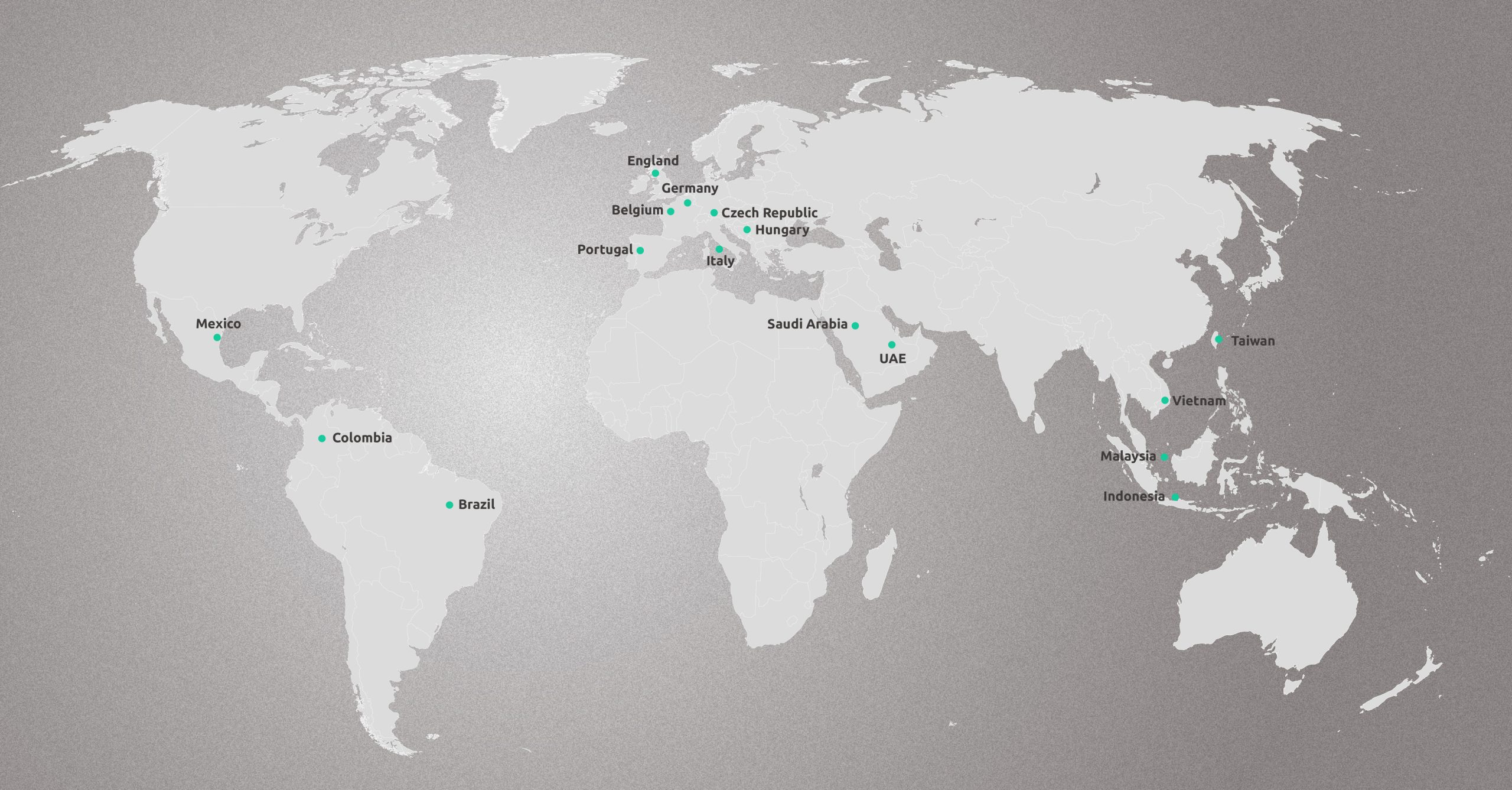
GLOBAL MARKET PRESENCE
Expanding global access to advanced spine care is at the core of Wiltrom’s mission—bringing safer, less invasive treatment options to patients everywhere.